When you pick up a prescription, you might see two options: the familiar brand name or a cheaper generic version. Many people wonder - does switching to a generic change how your meds interact with other drugs, food, or supplements? The short answer: no. The risk of drug interactions is essentially the same for generic and brand-name medications.
Why the confusion exists
It’s easy to assume that because generics cost less, they must be different. Maybe they’re weaker. Maybe they’re made with cheaper stuff. And if they’re different, maybe they cause different side effects or interactions. That’s a natural worry - especially if you’ve had a bad reaction to a medication before. But here’s what actually happens: generics aren’t copies. They’re required by law to be identical in one critical way - the active ingredient. That’s the part of the drug that actually works in your body. Whether it’s metformin for diabetes, lisinopril for high blood pressure, or sertraline for depression, the active ingredient in a generic is the exact same molecule as in the brand name. And that’s what determines how your body absorbs the drug, how it interacts with other medications, and what side effects you might get. The confusion often comes from the inactive ingredients. These are things like fillers, dyes, or preservatives - the stuff that holds the pill together or makes it look different. A brand-name pill might use cornstarch as a filler. A generic might use lactose. For most people, that doesn’t matter. But if you’re allergic to lactose or sensitive to certain dyes, you might notice a reaction. That’s not a drug interaction - it’s an allergy to an ingredient. And yes, that can happen with either brand or generic. It’s rare, but it’s not about the drug’s effect - it’s about your body reacting to something else in the pill.How regulators ensure safety
The U.S. Food and Drug Administration (FDA) doesn’t just approve generics because they’re cheaper. They have to pass strict tests. Every generic must prove it’s bioequivalent to the brand-name version. That means it delivers the same amount of active ingredient into your bloodstream at the same rate. The FDA requires that the generic’s absorption falls within 80% to 125% of the brand’s. That’s not a wide gap - it’s a tight range designed to make sure your body gets the same dose, every time. For most drugs, that’s more than enough. But for drugs with a narrow therapeutic index - like warfarin, lithium, or levothyroxine - the rules are even stricter. In those cases, the FDA requires the generic to match within 90% to 111%. These are the drugs where even a tiny difference in blood levels can cause problems. So if you’re on one of these, your doctor or pharmacist might monitor you more closely after a switch - not because generics are unsafe, but because precision matters. A 2020 study in Scientific Reports looked at 17 cardiovascular drugs and followed over 100,000 patients. It found that people taking generics had lower rates of heart attacks, strokes, and death than those on brand-name versions. That’s not because generics are better - it’s likely because more people could afford to take them consistently. But it does show that switching to generics doesn’t increase risk. In fact, it might improve outcomes.What the data says about interactions
The FDA’s own adverse event database, FAERS, tracked reports from 2015 to 2020. It found that 0.78% of brand-name drug users reported drug interactions. For generics, it was 0.82%. That’s a difference of 0.04 percentage points - not statistically meaningful. In plain terms: the risk is the same. A 2022 review in JAMA Internal Medicine summed it up: “The vast majority of evidence suggests that generic drugs are therapeutically equivalent to their brand-name counterparts, including regarding drug interaction profiles.” Even the American College of Clinical Pharmacology says bioequivalent drugs can be expected to have the same interaction potential. That’s the official stance from the experts who study how drugs behave in the body. So why do some people swear their generic made them feel different? Sometimes, it’s the placebo effect - but flipped. It’s called the nocebo effect. If you believe generics are inferior, your brain might interpret normal side effects as something worse. WebMD data shows complaints spike in the first few months after a new generic hits the market - then drop off. That pattern suggests people are more alert to side effects when they expect them.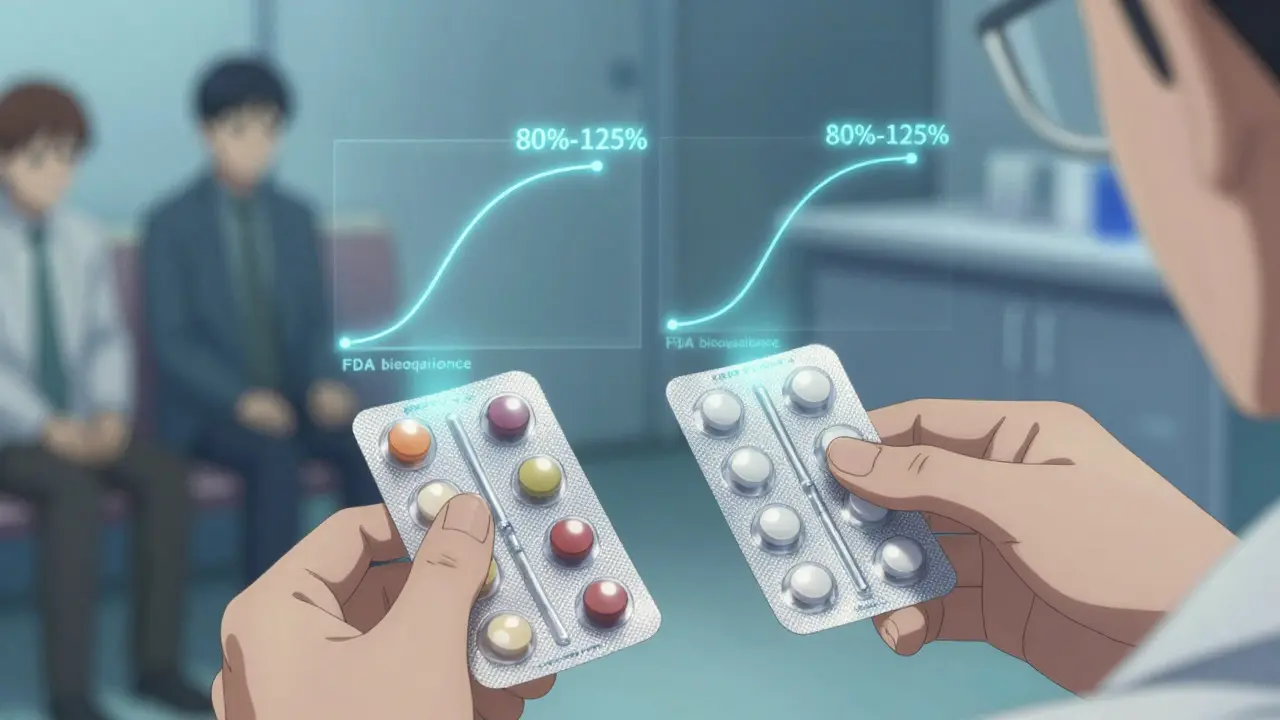
Real-world stories - what patients actually experience
On Reddit’s r/pharmacy community, a 2023 thread asked about generic vs. brand interactions. Out of 147 comments, 68% said they noticed no difference. Twenty-two percent reported changes - but many of those were about side effects like dizziness or nausea, not new drug interactions. One user mentioned increased drowsiness after switching from brand Ambien to generic zolpidem while taking sertraline. That’s a valid concern. But here’s the thing: zolpidem and sertraline interact regardless of whether the zolpidem is brand or generic. The interaction is between the two active ingredients, not the pill’s filler. Another user said their generic thyroid pill made them feel “off.” Their doctor checked their TSH levels - they were normal. The pill was fine. The issue? They’d switched brands three times in six months. Every time, they felt different. It wasn’t the drug - it was the change itself. Consumer Reports surveyed 1,200 people in 2022. Eighteen percent thought generics had different interaction risks. But only 4% had personally experienced a difference after switching. That gap tells you something: fear is common. Actual problems are rare.When you should be cautious
There are a few situations where extra care matters:- Narrow therapeutic index drugs: Warfarin, digoxin, phenytoin, levothyroxine. If you’re on one of these, your doctor might prefer you stick with one brand or generic. Consistency matters more than cost here.
- Allergies or sensitivities: If you’re allergic to lactose, gluten, or certain dyes, check the inactive ingredients. The FDA’s Orange Book now lists these for every approved generic. Your pharmacist can help you find one that’s safe.
- Multiple switches: Going back and forth between different generics can confuse your body. Stick with one version if it’s working. If you’re having issues, don’t assume it’s the generic - ask your pharmacist to check if the formulation changed.
What you can do
You don’t need to be a pharmacist to stay safe. Here’s what actually helps:- Keep a list of all your meds - including supplements and OTC drugs. Bring it to every appointment.
- Ask your pharmacist: “Is this generic the same as my last one?” If the pill looks different, they can confirm it’s the same active ingredient.
- If you notice new side effects after switching, don’t assume it’s the generic. Write down what changed, when, and how you feel. Talk to your doctor. It might be a drug interaction - but it’s likely unrelated to whether the drug is generic.
- Don’t avoid generics out of fear. They’re safe, effective, and save billions in healthcare costs every year. The FDA estimates they’ve saved $1.68 trillion in the U.S. over the past decade.
The bottom line
Drug interactions depend on the active ingredient - not the brand name. A generic drug is not a weaker version. It’s the same medicine, tested to the same standards, sold for less. The risk of interactions is not higher. In fact, because more people can afford to take them, generics may reduce overall health risks by improving adherence. If you’ve had a bad experience, it’s worth investigating - but don’t assume it’s because the drug is generic. Talk to your pharmacist. Check the ingredients. Monitor your symptoms. And remember: the science is clear. Generics work the same. They interact the same. And they’re just as safe.Are generic drugs less effective than brand-name drugs?
No. Generic drugs must contain the same active ingredient, strength, and dosage form as the brand-name version. They’re required to be bioequivalent - meaning they deliver the same amount of medicine into your bloodstream at the same rate. The FDA approves them only after rigorous testing. Studies show generics work just as well for conditions like high blood pressure, depression, and diabetes.
Can generic drugs cause different side effects than brand-name drugs?
The side effects from the active ingredient are the same. But sometimes, different inactive ingredients - like fillers or dyes - can cause rare reactions. For example, someone allergic to lactose might get stomach upset from a generic that uses lactose as a filler. That’s not a drug interaction - it’s an allergy to an ingredient. Your pharmacist can help you find a generic without that ingredient.
Do drug interactions change when I switch from brand to generic?
No. Drug interactions depend on the active ingredient, not whether the drug is generic or brand-name. If your medication interacts with alcohol, grapefruit juice, or another drug, that interaction remains the same regardless of the brand. The FDA and major medical groups confirm that bioequivalent generics have the same interaction profile.
Should I avoid switching to a generic if I’m on multiple medications?
Not necessarily. Most people switch safely. But if you’re on a narrow therapeutic index drug - like warfarin, lithium, or thyroid medicine - your doctor might prefer you stay on one version. Consistency helps avoid small changes in blood levels. For other drugs, switching is safe and often saves money. Always check with your pharmacist before switching.
Why do some people say their generic made them feel worse?
Sometimes it’s the nocebo effect - when you expect something to go wrong, your brain interprets normal sensations as side effects. Other times, it’s a change in inactive ingredients causing minor discomfort, like a different filler causing bloating. Rarely, it’s a formulation issue. If you feel different after switching, track your symptoms and talk to your pharmacist. Don’t assume the generic is unsafe - most of the time, it’s not the problem.
Are generic drugs tested as thoroughly as brand-name drugs?
Brand-name drugs go through years of clinical trials involving thousands of patients. Generics don’t need to repeat those. Instead, they prove bioequivalence - that they work the same way in the body. This process is shorter but still strict. The FDA requires generics to meet the same quality, strength, and purity standards. Thousands of generics are monitored after approval, and safety data shows they perform just as well.

I am a pharmaceutical expert with over 20 years of experience in the industry. I am passionate about bringing awareness and education on the importance of medications and supplements in managing diseases. In my spare time, I love to write and share insights about the latest advancements and trends in pharmaceuticals. My goal is to make complex medical information accessible to everyone.