By 2025, nearly 90% of all prescriptions filled in the United States were for generic drugs. In Germany, it was 88%. In India, the world’s largest supplier of generic medicines by volume, generics make up more than 70% of all drugs dispensed. Yet, the price of those same pills can vary wildly-from under $1 a month in India to over $20 in the U.S.-even when they’re chemically identical. Why? Because how countries handle generics isn’t about science. It’s about policy, power, and survival.
How Generics Work-And Why They Matter
Generic drugs aren’t knockoffs. They’re exact copies of brand-name medications, approved after patents expire. The active ingredient is the same. The dose is the same. The way it works in your body? Identical. The only difference? The price. Generics typically cost 80-90% less. That’s not marketing. That’s math. The U.S. saved $142 billion in Medicare spending in 2024 just from generics. That’s $2,643 per beneficiary. In the European Union, generics make up 65% of prescriptions but only 22% of total drug spending. In South Korea, a single policy change cut generic prices by nearly half. These aren’t small wins. They’re lifelines for patients with diabetes, high blood pressure, or depression who can’t afford their meds. But here’s the catch: making generics cheap doesn’t mean making them accessible. Some countries cut prices so hard that manufacturers stop making them. Others let prices stay high because their systems don’t push pharmacists to substitute. The real question isn’t whether generics work-it’s whether the system lets them work for everyone.The U.S. Model: High Volume, High Branded Costs
The United States leads the world in generic use. Over 90% of prescriptions are filled with generics. The FDA has approved more than 11,300 generic products. And yet, Americans still pay more for drugs than any other country. Why? Because generics aren’t the problem. Branded drugs are. The U.S. allows pharmaceutical companies to charge whatever they want for new drugs. A cancer pill might cost $15,000 a month. A generic version of the same drug, once it hits the market, might cost $30. But if you’re on a brand-name drug that’s still under patent, there’s no price control. That’s where the real cost piles up. The FDA’s Orange Book tracks every approved generic. The Competitive Generic Therapy (CGT) designation speeds up approval for drugs with little competition-like Zenara Pharma’s Sertraline capsules approved in August 2025. That’s a good thing. But even with fast approvals, development costs are still $5.9 million per product. And if the price you can charge after approval is too low, companies won’t bother. Pharmacy Benefit Managers (PBMs) make it worse. They control which drugs insurers cover. Sometimes, they charge higher copays for generics than for brand-name drugs. Patients see the same pill on the shelf, pay more for the cheap one, and get confused. Or worse-they skip their meds.Europe: Harmonized Rules, Fragmented Prices
The European Medicines Agency (EMA) approves generics for all 27 EU countries. But once approved, each country sets its own price. That’s where things get messy. Identical pills, same manufacturer, same batch-sold in Germany for €2.50 and in Bulgaria for €8.70. That’s a 248% difference. The OECD calls it a “bifurcated market.” It’s not just unfair. It’s inefficient. Manufacturers can’t plan. Pharmacies can’t stock reliably. Patients traveling between countries can’t refill their prescriptions. Germany uses mandatory substitution: pharmacists must switch you to a generic unless your doctor says no. Italy? No such rule. Only 67% of prescriptions there are for generics. The Netherlands uses a clever trick: they compare prices to non-EU countries like Norway and the UK, then pick the lowest. That’s how they keep prices down without direct price controls. The EU is trying to fix this. A new Pharmaceutical Package, expected in late 2025, aims to harmonize pricing rules and give faster market access to the first generic company to enter. But progress is slow. Politics, not science, still drives pricing.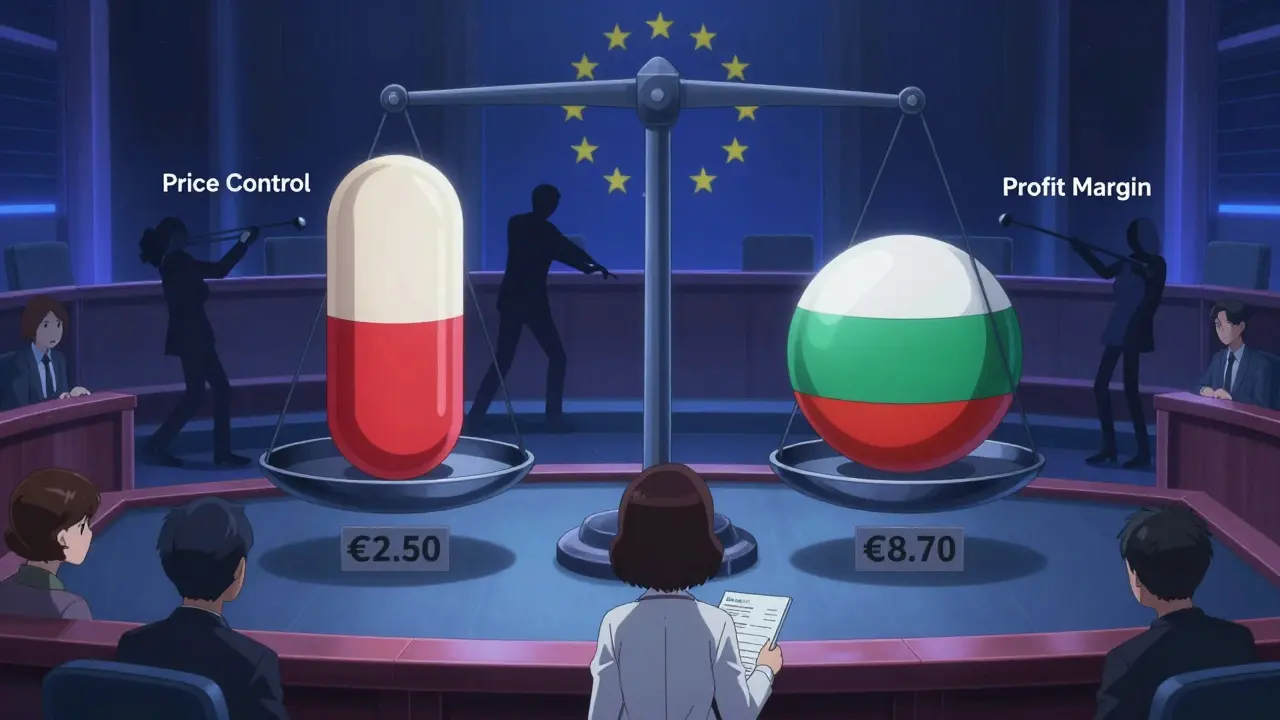
China’s Big Gamble: Buy in Bulk, Slash Prices
China’s Volume-Based Procurement (VBP) policy is the most aggressive experiment in generic pricing ever tried. Instead of letting hospitals negotiate one by one, the government gathers all public hospitals and says: “We’ll buy 80% of your demand for this drug. Bid lowest.” The results? Average price drops of 54.7%. Some drugs? Up to 93%. Amlodipine, a common blood pressure pill, went from $0.40 per tablet to $0.03. That’s incredible for patients. But it’s brutal for manufacturers. A 2025 survey by the China Generic Pharmaceutical Association found that 23% of companies were selling VBP drugs at a loss. Some stopped production. In 2024, 12 provinces ran out of Amlodipine for six to eight weeks. Patients waited. Doctors panicked. The government’s next move? Expand VBP to 150 more drugs in January 2026. Winning bidders must supply 80% of hospital demand at prices 65% below current levels. If manufacturers can’t make money, they won’t make pills. And if they don’t make pills, patients pay the price.India: The World’s Pharmacy, But With Quality Concerns
India makes 20% of the world’s generic drugs by volume. It’s the go-to source for low-cost medicines in Africa, Latin America, and Southeast Asia. That’s because India’s patent laws allow generic manufacturers to copy drugs even before patents expire-if they can prove the original drug isn’t “innovative” enough. But speed comes at a cost. Between 2022 and 2024, the FDA issued 17% more warning letters to Indian generic manufacturers for data integrity issues. That means falsified test results, missing records, or unsafe production conditions. One lab in Hyderabad was shut down in 2023 after inspectors found drug samples swapped out with water. Indian doctors report inconsistent bioavailability in generics for critical drugs like antiepileptics and blood thinners. One pill might work. The next batch might not. That’s not just inconvenient-it’s dangerous. Still, for millions who can’t afford branded drugs, India’s generics are the only option. The challenge isn’t access-it’s trust.South Korea: The Tightrope Between Competition and Quality
South Korea introduced its “1+3 Bioequivalence Policy” in 2020. Only three generic versions of a drug can be approved using the same bioequivalence data. After that, new entrants must prove their own data-costing millions. The goal? Stop the flood of low-quality, identical generics that drive prices down too far. The result? Fewer competitors. Fewer price drops. And a 29% drop in new generic launches between 2020 and 2024. They also created a pricing ladder: generics that meet both quality and price standards get 53.55% of the brand price. Those meeting only one criterion get 45.52%. The rest? Just 38.69%. It’s smart. It’s precise. But it also discourages innovation. If you’re a small manufacturer, why spend $5 million to prove your drug works if you’ll only get 38 cents on the dollar?
What Works? What Doesn’t?
There’s no one-size-fits-all model. But some patterns stand out:- High generic use? Look at the U.S., Germany, South Korea. All have strong policies pushing substitution.
- Lowest prices? China and the Netherlands. But China risks shortages. The Netherlands relies on clever pricing tricks that don’t always translate.
- Best quality control? The U.S. and EU have the strictest inspections. But even they struggle with global supply chains.
- Biggest risk? Price cuts so deep they kill manufacturing. When a company can’t make a profit, it stops making pills. Patients get left behind.
The Future: Patent Cliffs and Global Pressure
Between 2025 and 2030, branded drugs worth $217-$236 billion in annual sales will lose patent protection. That’s a goldmine for generics-if the system allows it. The U.S. Inflation Reduction Act will let Medicare negotiate prices on 10-20 high-cost drugs each year starting in 2028. That could cut branded drug revenues by 25-35%. That’s a wake-up call for pharma companies. They’ll push harder for generics to replace those drugs. Meanwhile, the International Generic and Biosimilars Association wants global standards for bioequivalence. Right now, a generic approved in the U.S. might not be accepted in Brazil or Nigeria. If countries recognized each other’s approvals, generics could reach low-income markets 18-24 months faster. But here’s the real test: Can we make generics affordable without making them unsafe? Can we let manufacturers survive while keeping prices low? The answer isn’t in a law. It’s in balance.What Patients Should Know
If you’re taking a generic drug:- It’s safe. The science is solid. Generics work.
- Check your insurance formulary. Sometimes, the brand-name drug costs less than the generic because of how your plan works.
- Ask your pharmacist: “Is this the same as the brand?” They can tell you.
- If your medication seems different-side effects, effectiveness-talk to your doctor. It’s rare, but it happens.
- Support policies that ensure manufacturers can make quality drugs at fair prices. Cheap isn’t good if it’s not safe.
Are generic drugs as safe and effective as brand-name drugs?
Yes. Generic drugs must meet the same strict standards as brand-name drugs for active ingredients, strength, dosage form, and how they’re absorbed by the body. The FDA, EMA, and other major regulators require bioequivalence testing-meaning generics must perform the same way in the body as the original. Millions of patients worldwide use generics safely every day. The only difference is the price and the packaging.
Why do some people say generics don’t work for them?
Rarely is it because the generic is ineffective. More often, it’s because of differences in inactive ingredients-like fillers or coatings-which can affect how fast the drug is absorbed. This matters most for drugs with a narrow therapeutic index, like warfarin or levothyroxine. If you switch generics and notice side effects or reduced effectiveness, talk to your doctor. You may need to stick with one manufacturer or switch back to the brand.
Why are generic prices so different between countries?
Because drug pricing isn’t based on production cost-it’s based on policy. Countries use tools like bulk purchasing (China), reference pricing (Netherlands), mandatory substitution (Germany), or price ceilings (South Korea). Some prioritize affordability over manufacturer profit. Others protect industry sustainability. That’s why the same pill can cost $0.05 in India and $5 in the U.S.-even if they come from the same factory.
Can generic drugs cause shortages?
Yes. When governments force prices too low, manufacturers stop making the drug because they can’t cover costs. China’s Volume-Based Procurement led to shortages of amlodipine and other essential medicines in 2024. The U.S. has seen shortages of antibiotics and chemotherapy drugs when profit margins collapsed. The solution isn’t higher prices-it’s smarter pricing that ensures manufacturers can stay in business.
Is it true that Indian generics are often unsafe?
Some are. The FDA has increased warning letters to Indian manufacturers due to data integrity issues-like falsified test results or poor quality control. But not all Indian generics are unsafe. Many are high-quality and life-saving. The problem is inconsistent regulation and pressure to cut costs. Patients in low-income countries rely on them, but they need better oversight-not bans. Global standards and better inspections are the answer.

I am a pharmaceutical expert with over 20 years of experience in the industry. I am passionate about bringing awareness and education on the importance of medications and supplements in managing diseases. In my spare time, I love to write and share insights about the latest advancements and trends in pharmaceuticals. My goal is to make complex medical information accessible to everyone.