Generic and brand-name drugs have the same active ingredients and carry the same risk of drug interactions. Regulatory standards ensure bioequivalence, making generics just as safe and effective as their branded counterparts.
When you pick up a generic version of your prescription, you might wonder: bioequivalence, the scientific standard that proves two drugs perform the same way in the body. Also known as therapeutic equivalence, it’s the reason your pharmacist can swap a brand-name drug for a cheaper copy without your doctor changing anything. This isn’t just paperwork—it’s your safety net. If a generic drug isn’t bioequivalent, it could be too weak to work, or too strong and cause side effects.
Think of it like this: two cars might look the same, but if one takes twice as long to go from 0 to 60, or burns fuel differently, you’d notice. Same with drugs. generic drugs, lower-cost versions of brand-name medications approved after the original patent expires must match the original in how fast and how much of the drug enters your bloodstream. That’s called absorption rate, how quickly the body takes in the active ingredient. And active ingredient, the chemical that actually treats your condition has to be identical in amount and form. If it doesn’t pass these tests, it doesn’t get approved.
This is why your warfarin, metformin, or ethambutol generic works just like the brand. The FDA and global regulators don’t just trust the manufacturer—they test it. Blood samples, controlled studies, strict limits on variation. That’s why switching from brand to generic for conditions like atrial fibrillation, diabetes, or tuberculosis doesn’t mean you’re taking a risk. It means you’re saving money without losing control.
But bioequivalence isn’t the same as interchangeability. Some drugs, like blood thinners or seizure meds, need extra care—even if they’re bioequivalent, your doctor might prefer you stick with one version. Why? Because tiny differences in how your body handles the drug can add up over time. That’s why INR monitoring matters for warfarin, and why Dong Quai can mess with your levels. Bioequivalence keeps the baseline steady, but your body’s response still needs watching.
What you’ll find below are real-world stories about how bioequivalence plays out in daily life. From how generic antibiotics like cefaclor compare to brand-name versions, to why switching from Combivir to newer HIV drugs isn’t just about cost but how your body absorbs them. You’ll see how drug interactions with minocycline or diclofenac SR can change if the formulation shifts, and why prescription assistance programs often rely on bioequivalent generics to keep people on treatment. This isn’t theory. It’s what happens in your body, your pharmacy, and your doctor’s office every day.

Generic and brand-name drugs have the same active ingredients and carry the same risk of drug interactions. Regulatory standards ensure bioequivalence, making generics just as safe and effective as their branded counterparts.
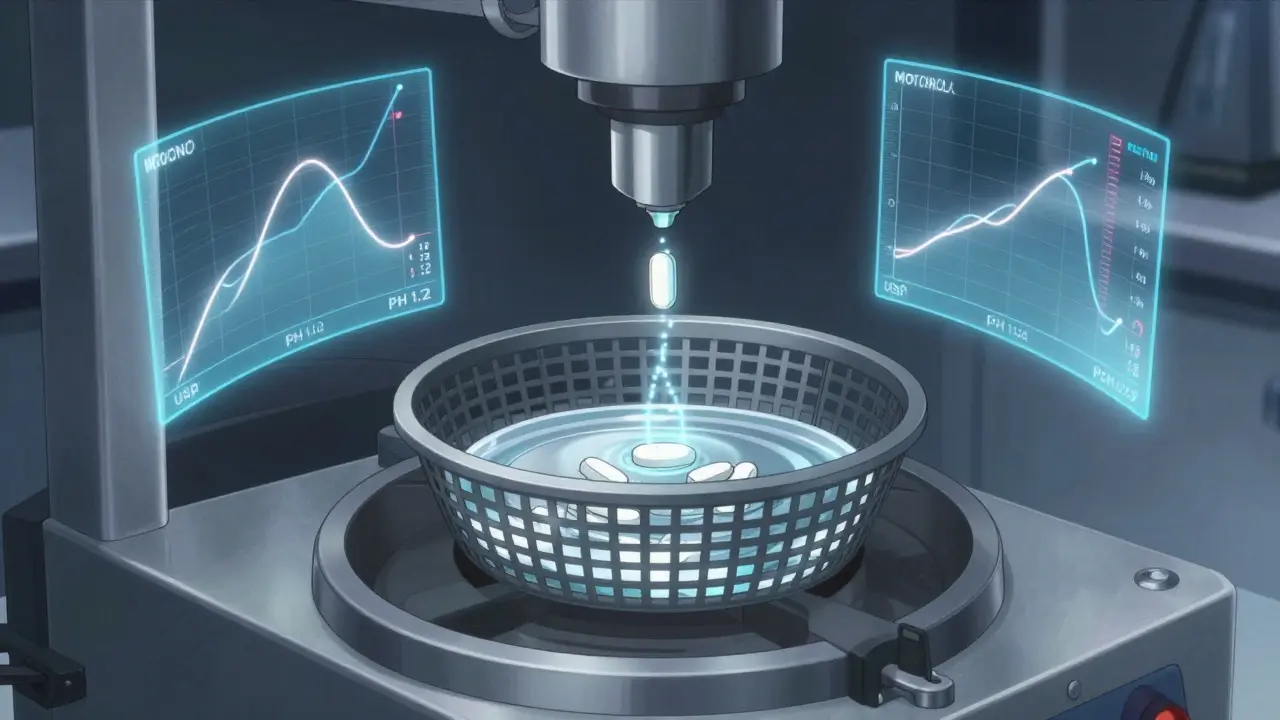
Dissolution testing is how the FDA ensures generic drugs release their active ingredients at the same rate as brand-name versions. It replaces human trials for many drugs, saving time and cost while maintaining quality.

NTI generics require strict regulatory oversight due to their narrow safety margin. This article compares how the FDA, EMA, Canada, Japan, and others regulate these high-risk drugs - and why global alignment is critical for patient safety.