
An ANDA is the FDA pathway that allows generic drugs to enter the market without repeating costly clinical trials. It saves billions annually and makes essential medicines affordable. Here's how it works, why it matters, and what you need to know.

An ANDA is the FDA pathway that allows generic drugs to enter the market without repeating costly clinical trials. It saves billions annually and makes essential medicines affordable. Here's how it works, why it matters, and what you need to know.
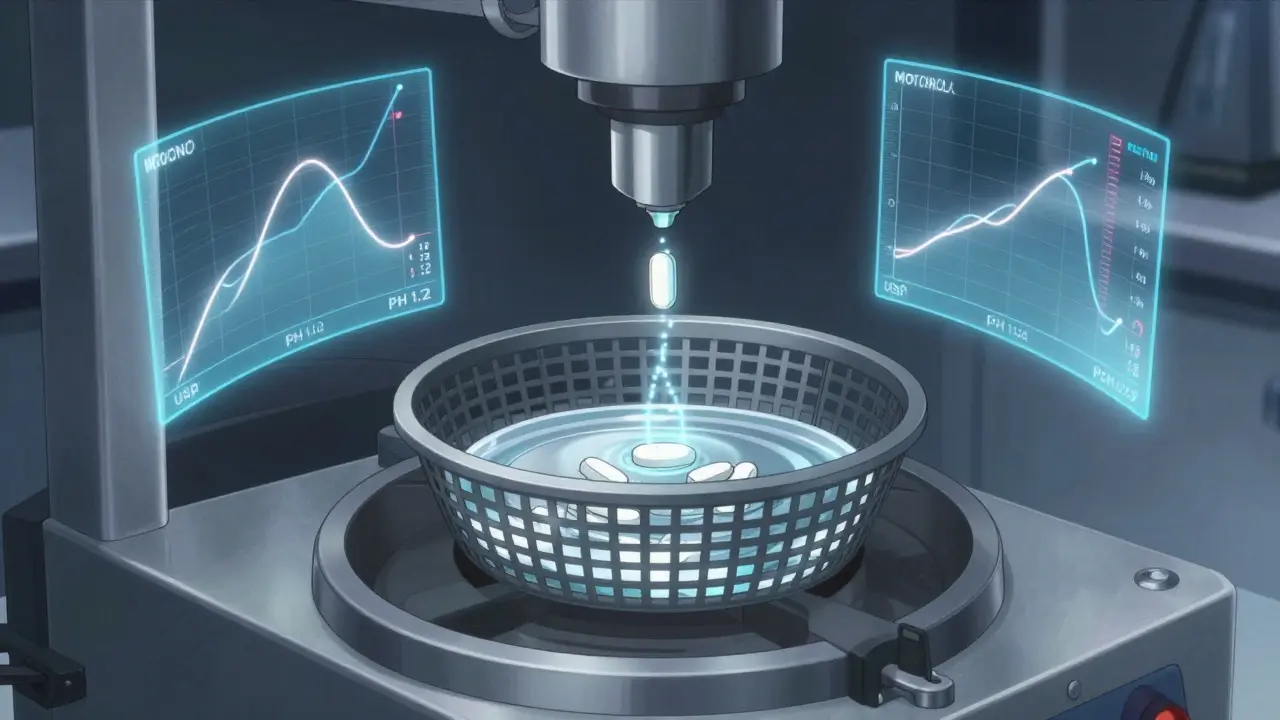
Dissolution testing is how the FDA ensures generic drugs release their active ingredients at the same rate as brand-name versions. It replaces human trials for many drugs, saving time and cost while maintaining quality.